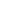Les Johnson and Joseph Meany
Carbon composites, carbon nanotubes, buckyballs—if you follow science news, you are bound to have read about these “wonder materials” and the promise they hold for finally enabling the science-fictional devices we fans have been reading about for decades. From space elevators to impenetrable body armor, there are abundant speculations that these dream devices will transition from science-fiction to science-fact within the foreseeable future. The announcement of each new wonder material brings with it abundant opportunities to realize far-flung hopes and fantasies. The 19th and 20th centuries gave us many wonder materials to transform our world. Many elements were found in the 19th century for the first time; aluminum began as a wonder material before eventually evolving into the abundant commodity it is today. Around the turn of the 20th century, plastics were invented for the first time and have come to irreversibly change the face of this planet. Liquid crystals shook up chemists by establishing that molecules can be liquid but still have order like normal solid crystals. High explosives and nuclear weapons changed politics forever. Now, there is another material that needs to be added to the list. One that has been all around us since our discovery of graphite (though we didn’t realize it), and is rapidly advancing from a topic within fundamental research to a material used in commercial products: graphene.
What would you think if I told you that hidden in ordinary graphite, the “lead” used to make pencils, is a material more than three hundred times stronger than steel and capable of carrying current nearly as efficiently as a superconductor? What if I told you that its discoverers initially isolated it using not much more than Scotch™ Tape and a tenacious graduate student? The flaky structure of graphene and graphite allowed them to be pulled gently apart, like pancakes off a stack.
The material, graphene, has an interesting and unique two-dimensional structure—it’s planar. Most molecules are three-dimensional in nature, having bonds that pop up and down, left and right. Carbon, in diamond, extends out in all directions (Figure 1). That is why it forms nice pretty stones for jewelers to shape, with cleavage lines according to the way each bond forms. Carbon’s four available bonds send out differently in graphene, where they only send out left to right. The atoms in graphene are bound to a flat surface (Figure 2).
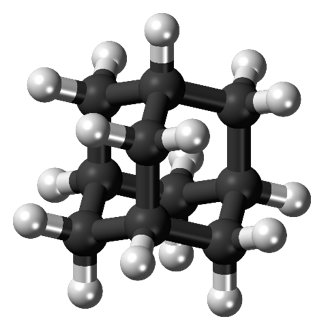
Figure 1 Adamantane, which is a single unit of diamond, is a cagelike molecule where carbon (black balls) extends in all directions. Extending adamantane out by replacing the hydrogen atoms (white balls) with more carbon, a diamond forms. Credit: https://commons.wikimedia.org/w/index.php?curid=20310245
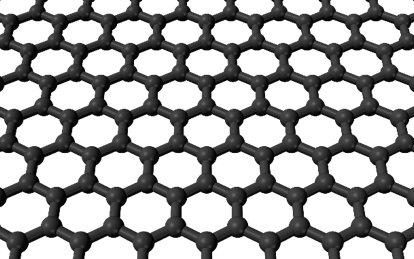
Figure 2 Graphene consists of only carbon atoms (black balls), bonded together in a flat ring shape, forming a single-atomic-layer thick sheet. Credit: https://commons.wikimedia.org/wiki/File:Graphen.jpg
It is this monoatomic layer of carbon atoms that gives graphene its interesting and potentially very useful properties. Before we delve into the many applications of graphene, we should first relay the story of its discovery, which led to its discoverers being awarded the 2010 Nobel Prize in Physics. In 2004, Andre Geim and Konstantin Novoselov of the University of Manchester (United Kingdom) turned the world upside down when they announced the discovery of the material that had long-been anticipated, but was considered by some experts impossible to isolate and analyze. Critics were stunned that the material could, in fact, exist and that they had found it in ordinary graphite. Like most academic researchers, the Geim group at Manchester had graduate students. Graduate students in a science department are often the unsung heroes of scientific research. They are the ones to take direction from their professors and perform the tedious, time-consuming work which is occasionally punctuated by the famous “aha!” moments of science after much crucial head-scratching. In this case, one of Geim’s graduate student (not Novoselov’s) was given the task of isolating a thin piece of graphite from a solid graphite slab—which he did, by polishing it to ever smaller sizes until only a fleck remained. They weren’t looking to make graphene. They wanted a small enough piece of graphite that could be used in research they hoped would lead to a new form of transistor. The polishing approach didn’t work well and caused Geim to think of alternative approaches.
A research colleague suggested they try using everyday office tape, sticking it to the surface of a brick of graphite and then pulling it away. Some of the graphite brick stuck to the tape and cleaved away from the brick to remove the impurities on the surface leading to two new pristine exposed sample surfaces that might be suitable for research. Only, instead of looking at the graphite brick surface that remained after taping and pulling, they decided to look at the graphite that had been pulled away. Repeated cleaving of this sample yielded thinner and thinner samples. They found not just dirt and debris stuck to the tape, but very small samples of an interesting form of graphite that, when placed under a sufficiently powerful microscope, was found to be a single layer thick sheet of carbon—they had finally isolated graphene.
Amazingly enough, you can repeat this process at home using a pencil and paper. Write yourself or your significant other a note and then use sticky tape on the penciled notes. When you remove the tape, you will also remove a thin layer of pencil lead (graphite) from the paper. If you were to look at this layer of graphite under a powerful microscope, then you would see that it also contains small scattered flakes of individual graphite plates, or graphene. Unfortunately, Andre Geim and Konstantin Novoselov already won the Nobel Prize for doing similarly so you can forget about your trip to Stockholm.
After graphene was isolated, Geim, Novoselov and other researchers around the world began looking at its physical and electrical properties. One obvious barrier came as they needed to search for other ways of making it. For any new material to be useful on an industrial scale, there must be a way to mass produce it. Using rooms filled with graduate students, piles of graphite, and lots of Scotch™ Tape is simply not going to produce graphene on a scale to be truly useful. Fortunately, researchers have found other ways to mass produce graphene and several companies are now selling it. Once it was shown possible to fabricate flaked graphene, the flood gates opened and people began making it all over the world. The popularity of graphene research is difficult to properly describe qualitatively. With the one-two punch from buckminsterfullerenes appearing in the late-1980s and the carbon nanotubes appearing in the mid-1990s, there was finally an abundance of funding pouring in to research unusual forms (allotropes) of this previously passé element. While many scientific discoveries are wrongly described as inevitabilities of their time, graphene research was already tantalizing enough around the turn of the millennium that attempts to isolate (and more importantly, characterize) graphene had already been underway for more than a decade before Geim and Novoselov stepped into the ring. Geim says that their primary contribution to the field was not, in fact, the Scotch™ Tape method. Rather, he asserts that the discovery of the Quantum Hall Effect within the graphite and graphene flake transistors led to the explosion in funding for the material. This explosion was rapid and permanently transformative.
In the time between 1986 (when “graphene” as a term was coined) and 2003, barely 800 academic papers had been published which mention the word within its text, much less focus on it as a topic (Figure 3). This number expanded to a rate of thousands of papers per year in the late-2000s, to literally tens of thousands of papers per year in the early- to mid-2010s. Soon, with this rate of growth expanding at an exponential rate, we will reach a point where more articles are written in a single year about graphene than have ever been written in all the years previous, combined. Manufacturers are not sitting idle.

Figure 3 Number of academic publications referencing “graphene” in their text, per year, between 1986 and 2016 as indexed by SciFinder. Credit: Joseph Meany
To understand the hype, consider some of the potential graphene applications being touted or in development. Let’s begin with its mechanical strength - nothing beats graphene. Mechanical engineers rate the relative strength of materials in Pascals, named in honor of the French physicist, Blaise Pascal. Structural steel has a strength of about 400,000,000 Pascals. Kevlar 375,700,000 Pascals. Graphene? 130,000,000,000 Pascals. If you got lost in all the zeros, here is the bottom line: graphene is at least 325 times stronger than steel by weight. It is also exceedingly lightweight. One square meter of graphene weighs 1000 times less than a one square meter piece of notebook paper! We will talk about the applications of this strength a little later.
Graphene is not a superconductor, but it is close. A superconductor is material that conducts electrical current with one hundred percent efficiency; there are no losses that cause heating, thereby allowing lossless power transmission across them. The problem is that most superconductors must be kept very cold to work and they typically cannot exceed some current limit without losing their superconducting properties. Graphene can carry electrical current at room temperatures with an efficiency much better than copper, the industry standard, or even silver, one of the best room-temperature conductors ever discovered. If a method of mass producing graphene wires can be found, major improvements in the efficiency of appliances, electronics, and even the power grid can be attained. When circuitry efficiencies can be enhanced in this way, it means that even paltry gains in energy production by renewable resources can have a massive economic benefit.
One of the most vexing problems facing the electronics industry today is energy storage. Battery technology hasn’t significantly changed in the last hundred years. Though efficiency has improved, storing electrical power in chemical batteries is perhaps the number one limitation facing both the portable electronics industry as well as utilities, who are looking for more efficient ways to generate, store, and distribute electrical power. Graphene has the potential (pun intended), to enable a different sort of battery, a capacitor, to store huge amounts of energy and release it quickly on demand—making it a supercapacitor.
A typical capacitor consists of two conducting plates separated a distance, d, by some sort of nonconductor, or dielectric (Figure 4). As the voltage difference between the plates increases, more energy is being stored. If the area of the conducting plates can be increased within a given volume, the capacitor can store more energy – making it a better battery. This is where graphene comes into use. Recall that graphene is conducting and very, very, thin. A capacitor consisting of multiple, stacked graphene plates can be packed into a very small volume, creating a high-energy density, high-power battery or supercapacitor. Graphene’s flexibility would allow manufacturers to make supercapacitors flexible—a massive hurdle in the way of making tablets and phones as thin as paper!
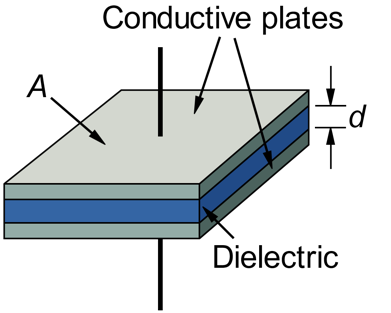
Figure 4 A capacitor stores energy in the electric field generated between two conducting places separated by a non-conductor, or dielectric. Credit: https://commons.wikimedia.org/wiki/File:Parallel_plate_capacitor.svg
Thanks to graphene’s structural strength and electrical conductivity, it is likely that cell phone manufacturers will soon be making their devices with transparent graphene screens that are much more scratch and break resistant than today’s state-of-the-art. Inside the cell phones will be graphene supercapacitor batteries and graphene wire traces instead of silver or copper. There is substantial effort being made to dope graphene with other atoms or molecules so that it can have semiconducting properties, making its use in portable electronics even more ubiquitous. This would be a boon for the world’s computer manufacturing industry, no longer needing to rely on mined heavy metals from politically sensitive locations and countries.
More mundane terrestrial uses of graphene are as structural reinforcement. We already discussed the mechanical strength of graphene when compared to steel and Kevlar so we should not be surprised to learn that among the first commercial applications of graphene are graphene-strengthened shoes, tennis rackets and automobile tires – all are items which tend to wear our or break easily. Next will be graphene-laced paints to provide additional strength to buildings and make them less susceptible to damage from debris during storms, or even hurricanes. A company in the UK has recently released an early version of graphene-based paint, a paint they are claiming helps with a building’s temperature regulation. While this is only a small step to the possible futures for graphene within home improvement, small but tangible increments are what will allow for consumers to grasp the true nature of this wonder material and drive the investment money into more research and development projects for even better products.
But is graphene only going to impact those rich enough to afford fancy belongings? After all, access to clean drinking water is a global concern and in some areas a true crisis. Graphene oxide, made from graphene by adding oxygen in various spots all over the graphene flake, has been shown able to filter polluted water, allowing only drinkable water to pass. Companies like Lockheed Martin are experimenting with graphene filters for use in water desalination plants and they claim to have a graphene-based process that is hundreds of times smaller and uses 99% less energy than conventional (non-graphene) approaches. The benefits of graphene will extend well beyond the high-tech world and into solving some of the most practical, and vexing, problems facing the world’s poor.
Graphene research occasionally takes detours into the realm of the bizarre. One group of Italian researchers decided to see if spiders who ingested graphene would produce stronger silk. They sprayed a group of spiders with a mixture of water, carbon nanotubes and small graphene particles. They then measured the strength of the silk the spiders produced and compared it with unsprayed spiders. The result? The strength of the silk increased dramatically—making it more than three times stronger than natural spider silk—even stronger than Kevlar. What about the spiders? Though some produced this super silk, several did not. They simply died. Think about it though, if an organism were selectively bred with an inherent resistance to graphene toxicity and a specific ability to produce super-strength graphene-enhanced natural silk, one could foresee a farming operation akin to the way that silkworms are used to create natural silk thread today. These threads may not be the lightweight tension cables of the future, but cloth spun with graphene has potential antibacterial properties.
This brings up the question of safety. Is graphene safe? Are we isolating and creating a super material that will become another asbestos? The answer is that graphene is safe for humans, with some caveats. Environmental protections today are far better than they were in decades past and there is already research being done to determine graphene’s toxicity. To date, it looks like graphene is relatively benign, unless you ingest or breathe it. Workers manufacturing piles of graphene flakes will still need the same protections they use around other fine particles. As with asbestos, a buildup of graphene in the lungs could cause long-term scarring and damage. As far as the environment goes, graphene seems to not have any long-term detriment to the environment, although research within that area is still weak. Fortunately, environmental persistence research performed for carbon nanotubes crosses over and is applicable to graphene! It has been found that in the environment, graphene can react with oxygen in the air to become pitted and malformed; this causes graphene to transform into graphene oxide, and graphene oxide can be broken down by microbes in the environment. In this way, graphene flakes are eventually chewed up and spit out by the bacteria, returning to the air as carbon dioxide. Early research shows that when certain molecules are added onto the graphene flake, they can make the flake deadlier than a bare, unreacted flake. It is important to note, though, that this research is still early and the reasons why the new molecule makes a difference is still unknown.
So, aside from soon being in just about every consumer product we buy or touch, how is graphene going to enable the science fictional future so many of us envision?
Let’s talk space elevators. I (author Johnson) am not personally a fan of space elevators (to learn why, check out my Baen essay, “http://www.baen.com/spacetethers”) but should one be built, it is likely to be made in part from graphene and related materials. Imagine catching an elevator from the surface of the Earth and taking it up 22,000 miles to Geostationary Earth Orbit (GEO). This would give us a way to carry people and cargo to space without the use of a rocket and at an incremental cost of electricity so low as to be nearly inconsequential (Figure 5). This idea, commonly referred to as a space elevator, or elevator to space, has been a dream of space advocates for decades. One of the most significant challenges to building a space elevator has been one of materials science: we simply did not have any materials strong enough to sustain their own weight in lengths approaching tens of thousands of miles. Now, thanks to Geim and Novoselov, we might.

Figure 5 Space elevators would can carry cargo and people from the surface of the Earth all the way to geostationary Earth orbit. Credit: https://commons.wikimedia.org/wiki/File:Nasa_space_elev.jpg
Graphene might also be a useful material for extremely large solar and laser sails. Solar sails are large, lightweight reflective fabrics attached to a spacecraft that provide propulsion by reflecting sunlight. Traditional nautical sails reflect wind, consisting of air molecules but solar sails reflect sunlight, which is made of light particles called photons. Like reflected air, sunlight transfers some of its momentum to the sail, causing it, and the spacecraft attached to it, to move. Solar sails are useful because they require no fuel and derive all their propulsion by reflecting light. The larger and lighter weight the sail, the more useful it is as a propulsion system. Current technology sails are less than 1000 square meters in area and weigh about ten to fifteen grams (about a tablespoon of butter) per square meter (about the size of half a door).
If we could build very large solar sails, perhaps square kilometers in area, and keep them lightweight, then we would have the materials we need to create truly solar sail propelled spacecraft. These craft would be capable of carrying humans throughout the inner solar system, or sending our robotic probes beyond the outer edge of the solar system. This is, of course, where graphene might come into play. Using graphene, it should be possible to build sails that are tens, if not hundreds, of square miles in area without incurring a damaging tear. Once deployed, these massive sails could easily undergo the mechanical stresses induced by being accelerated using sunlight or a combination of sunlight and laser light. The catch? Graphene is not reflective, so some sort of coating will have to be applied for this to work. The coating will inevitably make the sail more massive, but not significantly so. A realistic goal for researchers to strive for would be between 5x – 10x the weight of a bare graphene sail.
Graphene might also be used to make a lightweight, extremely tough body armor for soldiers and police. Both already use vests made from Kevlar to stop bullets and shrapnel, but these vests tend to be heavy and bulky. They also don’t provide full body protection because making and wearing a complete set of body armor is currently impractical. Police can’t do their work properly if they are dressed like medieval knights! But it might not be so with graphene. Recall that graphene is both two hundred times stronger than steel and weighs very little. A single layer blanket of graphene laid across a football field would weigh about one gram! With materials like this, full body armor suddenly becomes both possible and, most likely, inevitable. Watch out, Iron Man! While adding graphene to other materials to make it a composite, the composite does not directly take on the properties of graphene when it is introduced into the mix. Hopefully someday, we will move toward materials which can fully leverage the superlative nature of graphene’s properties.
Other potential applications of graphene include:
- Precision electrodes for creating and testing human neuron-to-computer interfaces without many of the side effects of state-of-the-art approaches. This could lead to cures for many neurological disorders, human thought-to-computer control for those that are physically disabled or fighter pilots trying to reduce the time to send commands to their aircraft by a few extra fractions of second.
- Lubricants to replace conventional graphite, extending the life and improving the performance of just about any type of machine, from next-generation turbines to skate boards. Mixing graphene with other materials, such as little balls of nanodiamonds, has proven to create interesting results. It seems that friction can be reduced almost to zero when graphene sheets are wrapped around the little nanodiamonds. The whole field of tribology is focused on the study of friction, and nanomaterials have opened up a wellspring of new research opportunities to try to control or minimize friction.
- Solar cells produce electricity when they interact with sunlight. Researchers in China have created graphene cells that can generate electricity from rainwater with an efficiency of just over six percent. With testing, these efficiencies will improve, potentially providing a way for regions of the world where solar power is impractical to generate power on rainy days, supplementing what they are able to do when the sun is not shining.
With so many potential commercial products being conceptualized and even prototyped, it should come as no surprise to learn that graphene-related patents are being filed by the thousands. In 2014 the patent office in the United Kingdom published a report that described the “gold rush” toward patenting just about every possible graphene application (Figure 6). As you can see in the data, the number of graphene-related patents filed is increasing yearly. This is a clear indication of where countries, companies and universities are investing their research and development funding – in graphene research!
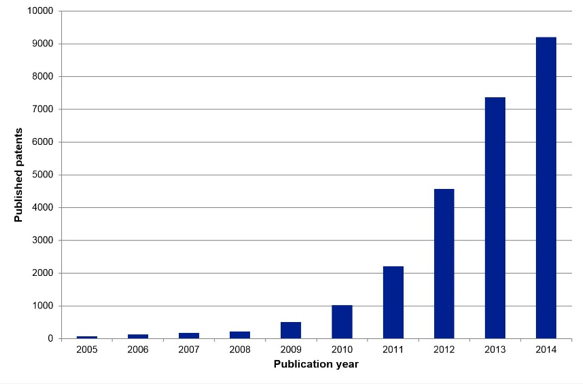
Figure 6 The number of graphene patents filed each year is increasing rapidly. Patent filings are often an indication of the amount of money being invested in a technology. (Image courtesy of the United Kingdom Intellectual Property Office.)
Graphene has been around for centuries, undetected, literally under our very noses, until 2004. The potential applications are many, cross many disciplines and fields of study and may very well change just about every aspect of our lives. It is THE wonder material of the 21st Century. And it is coming, soon, to touch your life in big ways and in the very small.
Copyright © 2017 Les Johnson and Joseph Meany
Les Johnson is a Baen science fiction author, popular science writer, and NASA technologist. Mission to Methone, Les’s debut “big science” science fiction novel will be available in February 2018. He is the author of On to the Asteroid and Back to the Moon with Travis S. Taylor, and Rescue Mode with Ben Bova, and numerous general science publications. To learn even more about graphene, watch for the release of Graphene: The Superstrong, Superthin, and Superversatile Material That Will Revolutionize the World by Les Johnson and Joseph Meany (to be released by Prometheus in February 2018). To learn more about Les, please visit his website at www.lesjohnsonauthor.com.
Joseph Meany, originally from Keene, NH, is a chemist whose research focuses on the design of molecules for nanomaterials in tiny electronic circuits. Space exploration and science fiction were Joseph's inspiration to become a scientist. In his spare time, he enjoys beer brewing and attending SF conventions.